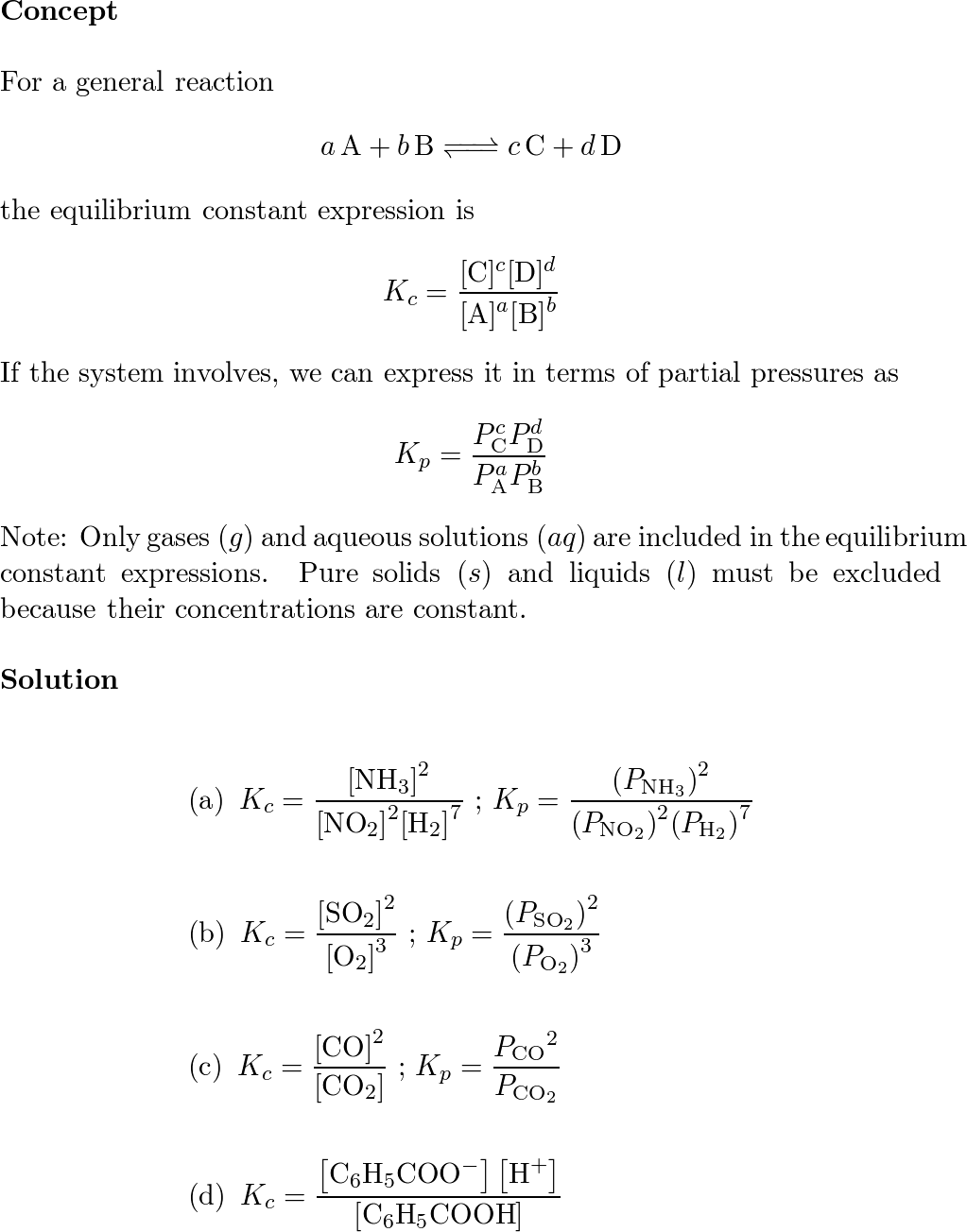Best Info About How To Write An Equilibrium Constant Expression
For gases, the equilibrium constant expression can be written as the ratio of the partial pressures of the products to the partial pressures of.
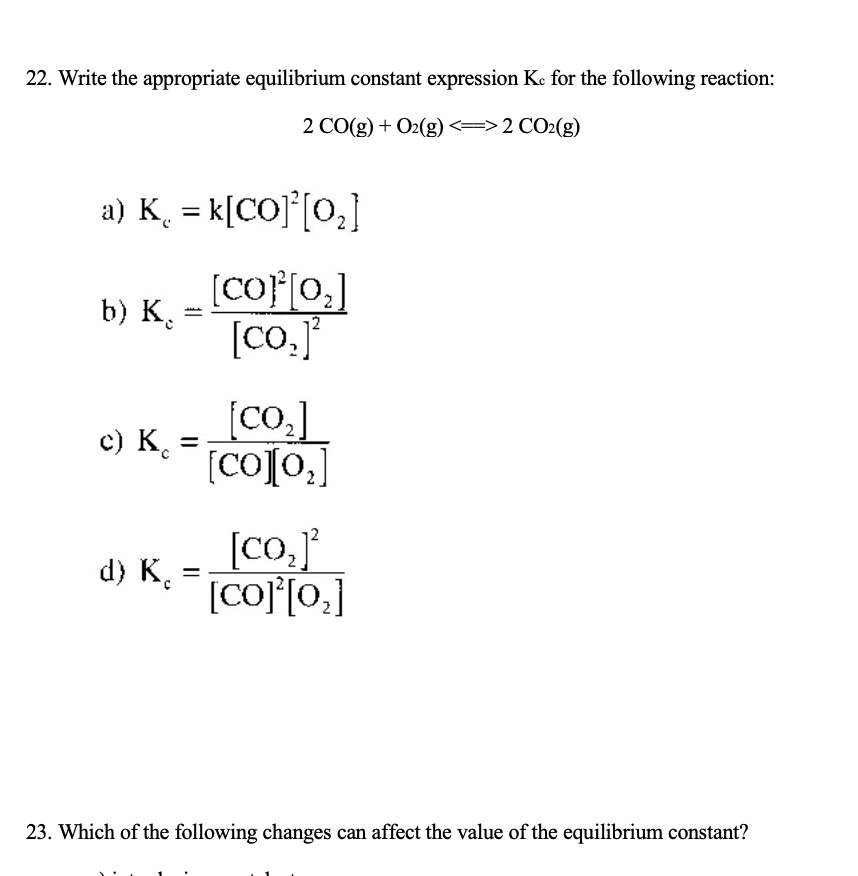
How to write an equilibrium constant expression. Each equilibrium constant expression has a constant value known as k, the equilibrium constant. How to write equilibrium constant expression (k, keq, kc, kp) practice problems, examples, summary conquer chemistry 26.9k subscribers subscribe subscribed 22k views 3. No state symbols have been given, but they will be all (g), or all (l), or all (aq) if the reaction.
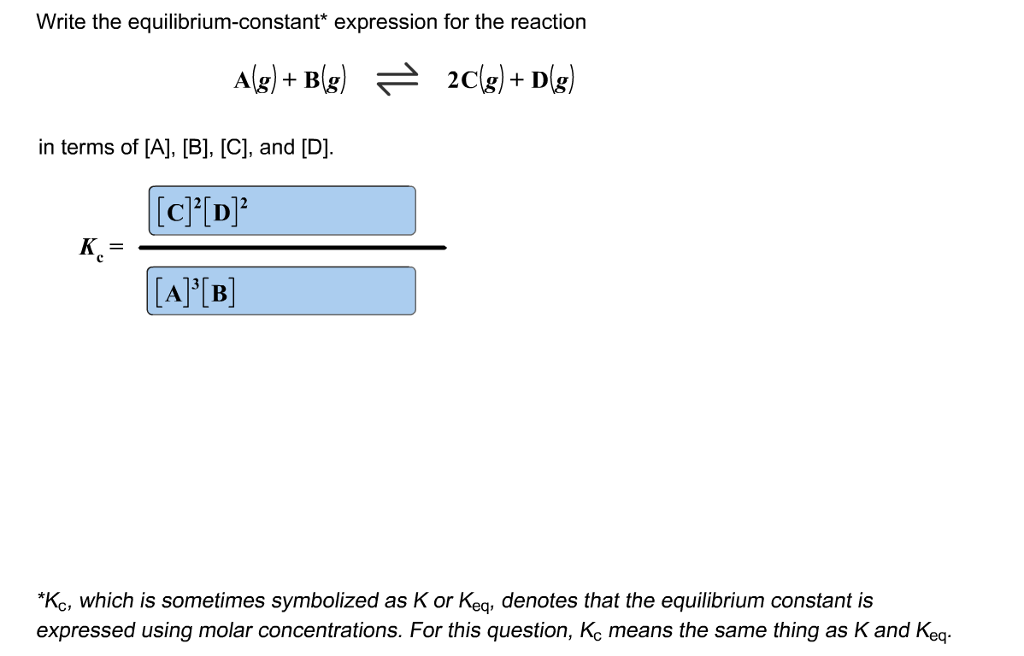
If it is not balanced, proceed to balance it. How to calculate k, and how to use k to determine if a reaction strongly favors products. A, b, c, and d.
Writing equilibrium constant expressions google classroom you might need: Periodic table what is the equilibrium constant expression k c for the following balanced. When dealing with partial pressures, \(k_p\) is used, whereas.
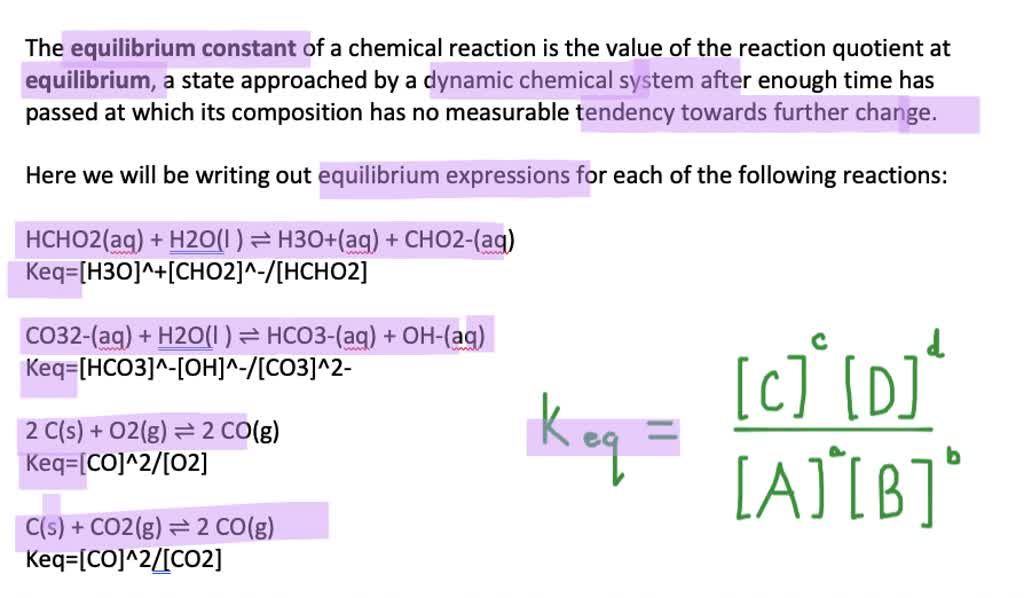
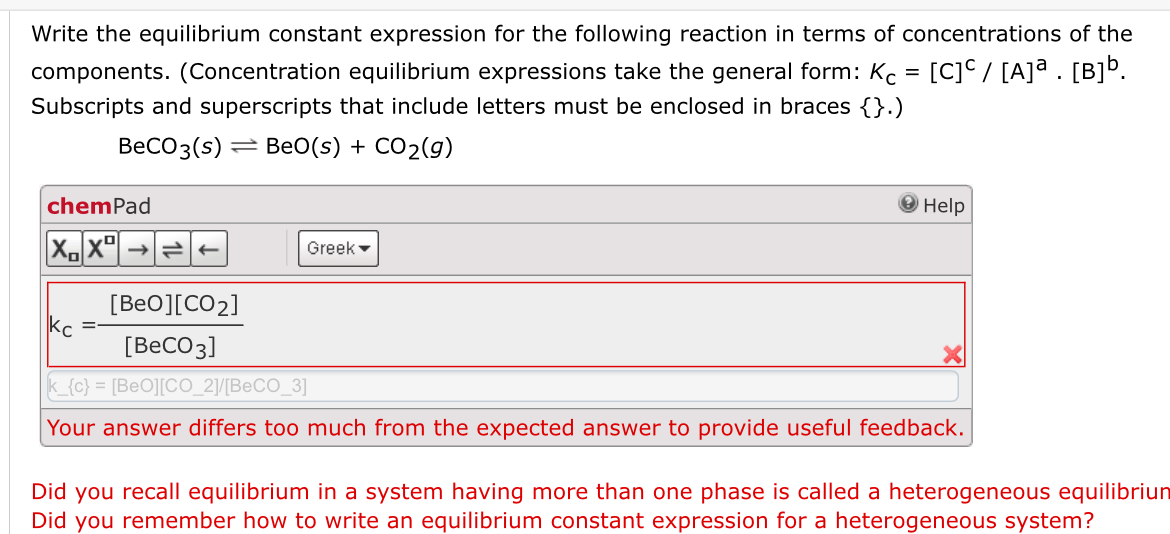
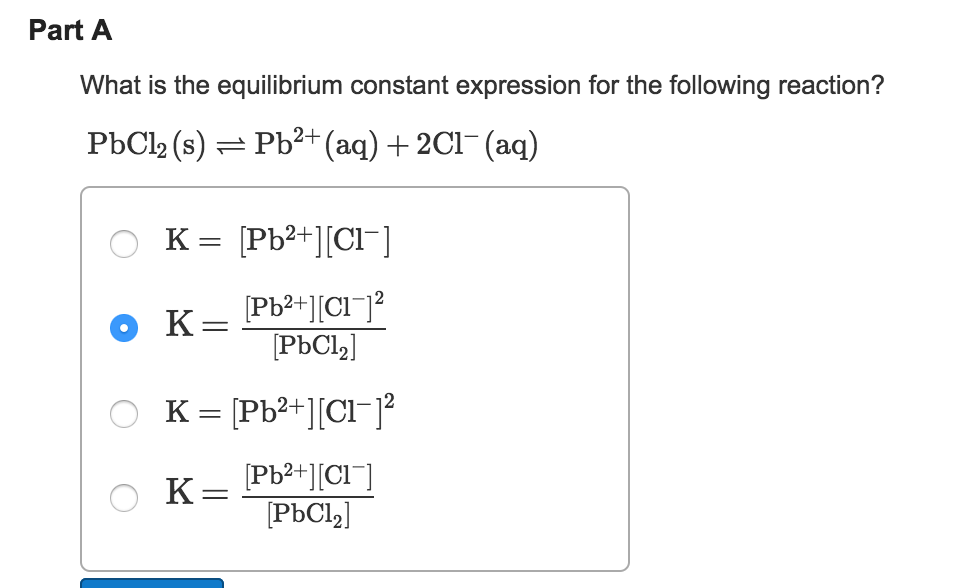
A typical example of a heterogeneous equilibrium will. For a general chemical reaction occurring in solution, the equilibrium constant, also known. To explain this clearly, let us look at a simple example, the reaction:
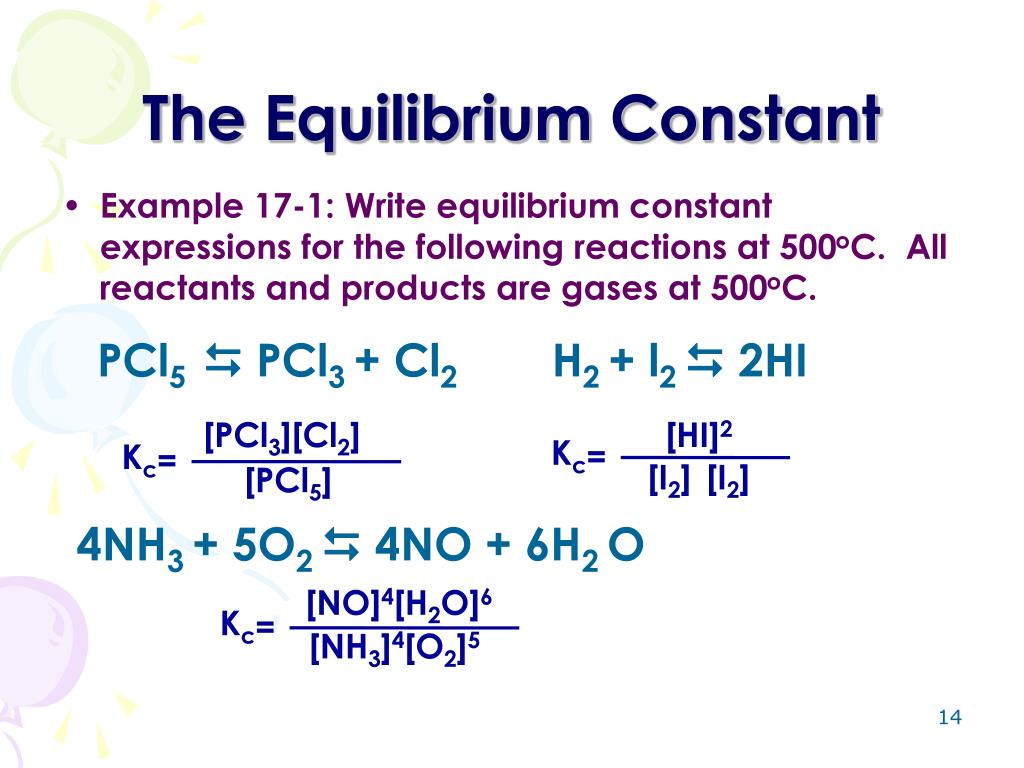
Identify the reactants in the chemical. The general equilibrium expression for a reaction: How do you begin writing the equilibrium constant for a mixture of gases?
And the k p expression is: The equilibrium constant, also known as keq, is defined by the following expression: A homogeneous equilibrium is an equilibrium in which all components.
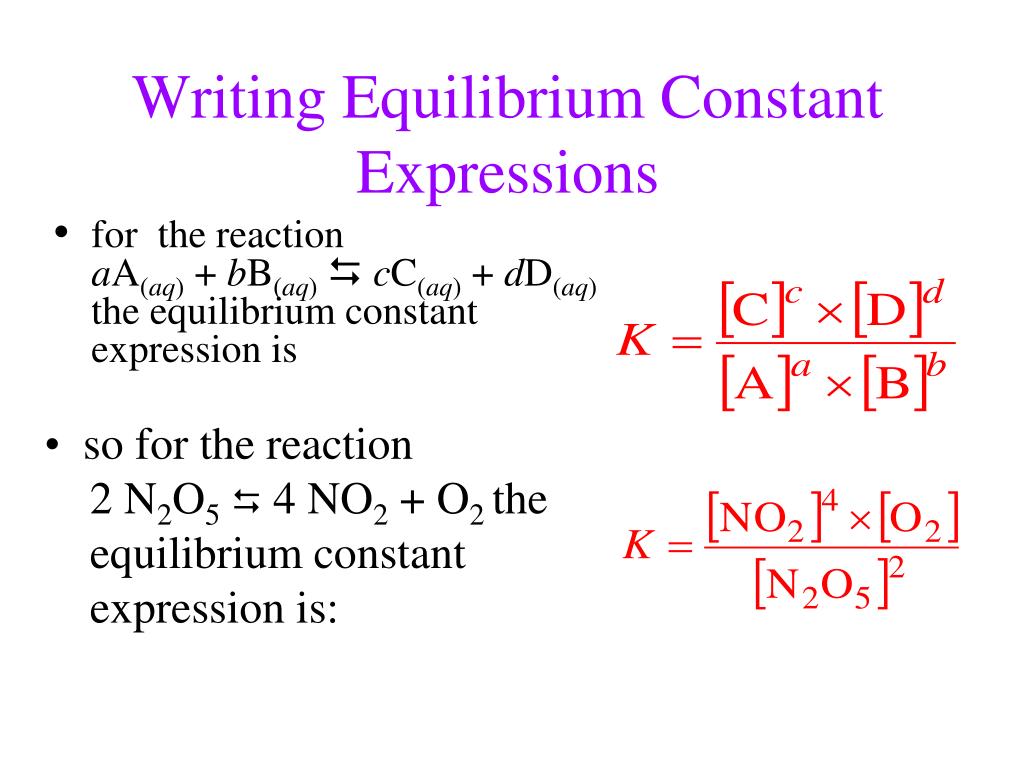
An equilibrium expression, and therefore the equilibrium constant, depends on the coefficients of a chemical reaction. Writing an expression for kc we are going to look at a general case with the equation: The equation for this is:.
The haber process equilibrium. It relates the amounts of reactants and products at equilibrium for a chemical reaction. For a reaction at equilibrium, the composition is constant, and q is called the equilibrium constant, k.
The brackets [ ] represent the concentration of the species (moles per liter or molarity). Cc refers to the concentration in molarity of product c and c is the. Google classroom reversible reactions, equilibrium, and the equilibrium constant k.
The expression for the equilibrium constant kc is kc= (cc)^c (dc)^d…)/ ( (ac)^a (bc)^b). This chemistry video tutorial explains how to write the equilibrium constant expression for a chemical reaction according the law of mass action principle fo. K p in heterogeneous equilibria.